Highlight ltamo28
New polymorphs of AM2O8 Materials
Cubic AM2O8 materials show the fascinating property of isotropic negative thermal expansion over a remarkably wide temperature range. They thus have a variety of potential applications, the most obvious being as components of composite materials with controllable positive, negative or even zero coefficients of thermal expansion. The synthesis of these materials is complicated by the fact that they are generally only metastable at room temperature and, in the case of ZrMo2O8, at the temperatures required for synthesis using “traditional” solid state techniques.
Angus Wilkinson and co-workers first showed how ZrMo2O8 could be prepared using low temperature precursor routes (C. Lind; A. P. Wilkinson; Z. B. Hu; S. Short; J. D. Jorgensen, Chem. Mater., 1998, 10, 2335). We’ve studied this process in depth by in-situ laboratory variable temperature powder diffraction. By doing this we’ve managed to identify and solve the structure of a new polymorph of AMo2O8 – the so called LT phase.
By solving the structure of this phase we’ve been able to rationalise the mechanism by which the precursor phase transforms to LT-ZrMo2O8 and suggest why LT-ZrMo2O8 transforms to cubic ZrMo2O8 rather than the more thermodynamically stable trigonal material – such detailed mechanistic insight into a solid state reaction is rare.
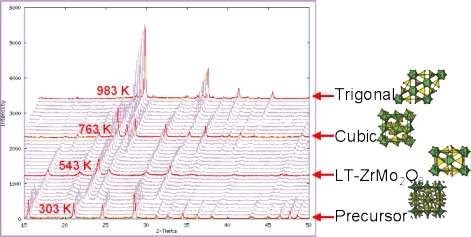
Figure: In-situ x-ray powder diffraction studies showing the transformation of precursor ZrMo2O7(OH)2.2H2O to LT-ZrMo2O8, cubic ZrMo2O8 then trigonal ZrMo2O8 with increasing temperature.
We’ve also studied the thermal expansion properties of the LT material and shown that it displays a bulk volume contraction with temperature and is itself a new NTE material.
Further details of this work can be found in the publication below:
S. Allen; N. R. Warmingham; R. K. B. Gover; J. S. O. Evans, “Synthesis, Structure and Thermal Contraction of a New Low Temperature Polymorph of ZrMo2O8”, Chemistry of Materials, 2003, 15, 3406-3410.
